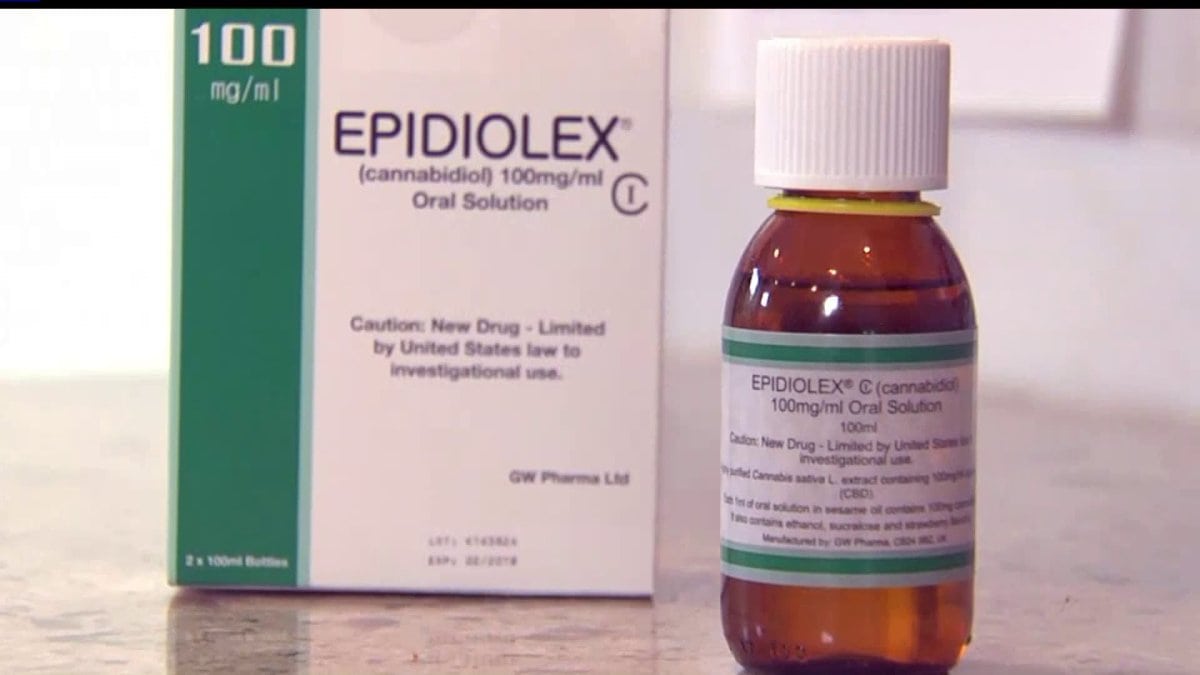
Today was a big day in the world of cannabis. The first ever plant-based pharmaceutical drug made from cannabis rich in cannabidiol (CBD), Epidiolex, was scheduled by the DEA. You’d think it would be scheduled as a II or III because “marijuana” is schedule I (no medical value with high potential for abuse/federally illegal). Not the case. Epidiolex was scheduled as level V the lowest possible schedule – least restrictive – which legitimizes the proven health benefits of CBD and its low potential for abuse. To give you a comparison, Epidiolex is in the same category as cough syrup with small amounts of codeine and yes, it requires a prescription. The FDA approved the drug in June for two rare and severe forms of epilepsy, Lennox Gastaut Syndrome (LGS) and Dravet Syndrome. It is possible that doctors will write prescriptions “off-label” for other epilepsy conditions that can benefit from CBD.
There is a lot of confusion surrounding this topic so we wanted to clarify a few things for you. As we learn more we will update this article. First, this doesn’t change anything for those of you who are using whole plant botanical extracts made from industrial hemp. The molecule CBD did not get scheduled today, only the drug Epidiolex. This motion by the DEA does allow for future FDA approved cannabis-derived pharmaceuticals as long as the THC limit is less than 0.1%. CBD did not get patented, you can’t patent a plant. CBD is not illegal if you are following your state laws, plus we have the added protection from the Farm Bill and Omnibus Bill from federal prosecution. Accessing CBD is a personal decision and you should make the best decisions to improve the quality of life for you or your loved one.
Currently, there are synthetic (man-made) forms of cannabis pharmaceuticals Cesamet and Marinol on the market which are scheduled as II and III and are prescribed to chemotherapy patients with vomiting and severe nausea. These drugs are THC based, not high in CBD. If you live in a medical or recreational cannabis state you can access THC products under state laws, although it is still federally illegal with “marijuana” as schedule I.
Having Epidiolex on the market is a move in the right direction because the US government clearly recognizes the medicinal benefits of cannabis. FDA approval also means insurance companies should cover the cost because it is expected to be pretty pricey. If they don’t (and you may need to fight for coverage)this may turn into an unaffordable option.
Fun Fact:
Did you know cannabis was available as a pharmaceutical drug in the US until 1947? Then reefer madness happened and the public was largely misinformed. We are thrilled cannabis is getting her positive reputation back!
Did you know:
We are conducting the largest observational cannabis study in the US in collaboration with Johns Hopkins University. In our epilepsy population, the average dose of CBD is 2mg/kg (the range is 7mg CBD-600mg per day). The starting dose of Epidiolex is 5mg/kg. Their studies titrated participants up to 20mg/kg or higher. It is really important to know and discuss this huge variance with your doctor.
If you found this article interesting, please share it with your friends and comment below!
[youtube https://www.youtube.com/watch?v=IJNLGIr37Ls]