At Realm of Caring, we believe in meeting people where they are, whether they’re exploring cannabis for the first time or looking for new options when other methods fall short. Suppositories are one such option, and while they may not be mainstream yet, they’re offering meaningful relief to people of all genders.
CBD and Cannabis Suppositories: A Groundbreaking Option for Targeted Relief
When we talk about cannabis wellness, most people think of tinctures, gummies, capsules, or inhalation. But there’s a lesser-known method that’s gaining attention for its effectiveness, especially when targeted relief is needed: suppositories.
What Are Suppositories?
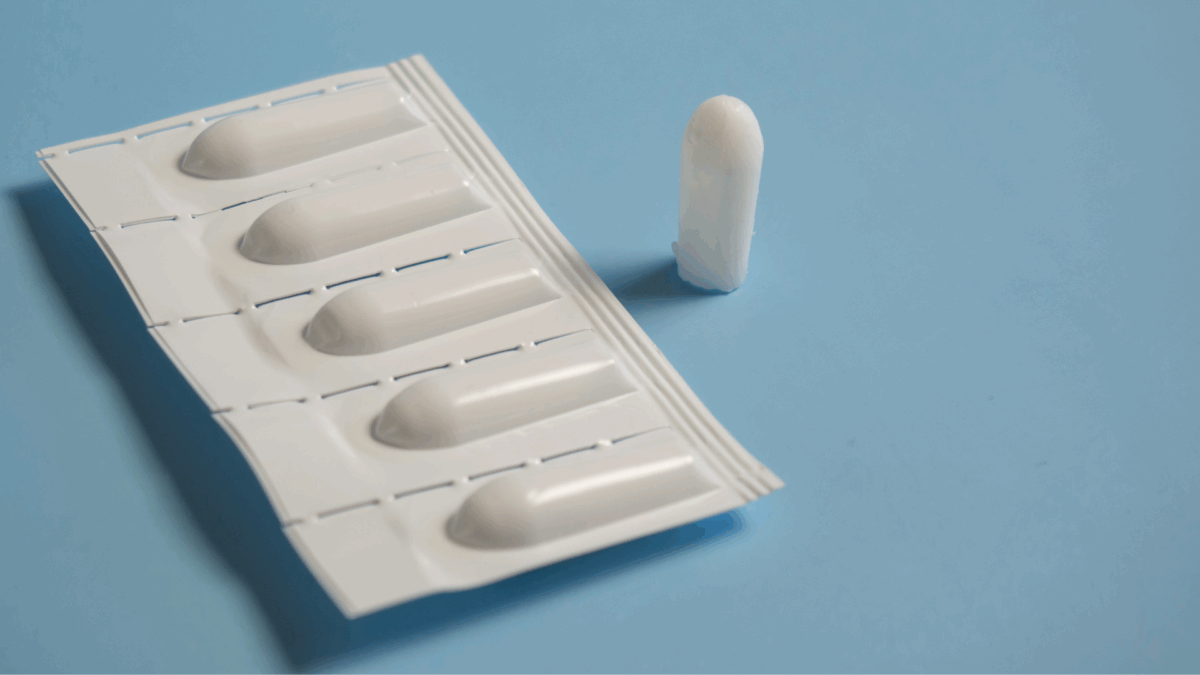
Cannabis suppositories are small, moldable forms, typically made with cocoa butter, that are inserted vaginally or rectally. They contain cannabinoids like CBD, THC, or a combination, and are designed to melt and absorb into nearby tissues, delivering effects locally and systemically.
Unlike oral methods, suppositories bypass the digestive system and liver, allowing for higher absorption and fewer psychoactive effects, even when THC is present. This makes them an appealing option for those who want the benefits of cannabis without the “high.”
Why This Method Matters
Cannabinoids delivered via suppositories may provide faster relief and better bioavailability than oral consumption. The mucosal tissues in the rectum and vagina are rich in blood vessels, which means cannabinoids can enter the bloodstream directly.
This method can be especially beneficial for those with:
- GI sensitivities
- Difficulty swallowing or digesting oral products
- A need for local, pelvic-area support
Benefits for Women
Women are finding cannabis suppositories helpful for a wide range of concerns:
- Menstrual cramps and bloating
- Endometriosis, a condition that often goes underdiagnosed and undertreated
- Sexual health, helping with lubrication, tension, and relaxation
- Menopause symptoms, including dryness, anxiety, and insomnia
By applying cannabinoids directly where inflammation and tension occur, some women report profound and fast-acting relief, often within 15 to 30 minutes.
Benefits for Men
While less frequently discussed, men are also benefiting from rectal cannabis suppositories. This method may help:
- Prostate and pelvic inflammation
- Rectal discomfort (including from hemorrhoids or IBD)
- Chronic pain and insomnia
For men seeking non-oral options or those navigating conditions like prostate cancer or GI disorders, suppositories offer a discreet, non-invasive, and potentially powerful therapeutic route.
Considerations Before You Try
Suppositories may be unfamiliar to many, so education is key:
- Start low and go slow – especially if THC is included
- Use clean hands or gloves for insertion and lie down for optimal absorption
- Store products in a cool, dark place to maintain integrity
- Speak to your healthcare provider if you’re undergoing other treatments or using prescription medications
As always, Realm of Caring is here to guide you with science-backed resources and personalized support.
Looking Ahead: The Need for Research
While anecdotal evidence is promising, there’s still much to learn. Realm of Caring is committed to supporting the research that will better inform use, access, and policy around cannabis therapies, including suppositories. We encourage curious consumers and medical professionals alike to stay engaged as the science evolves.
Join a Realm of Caring study today!
Final Thoughts
Suppositories may not be the first thing you think of when you consider cannabis therapy, but for many, they’ve become a game-changer.
If you’re looking for localized relief without systemic side effects, or you simply want to expand your wellness toolkit, suppositories might be worth exploring.
We’re here to answer your questions, connect you with the right resources, and make sure that everyone – regardless of gender, condition, or background – has access to safe, informed, and empowering plant-based care.