Our collective knowledge of the benefits of the cannabis plant for epilepsy continues to increase year after year. In addition to seizure reduction, we have learned through the literature that cannabis use may be attributed to an increase in the overall quality of life for one living with an epilepsy diagnosis. These improvements can range from decreased side effects of pharmaceuticals to better sleep and lessened anxiety.
It has been discovered that approximately one-third of individuals living with epilepsy do not respond well to conventional antiepileptic drugs (AEDs) and are therefore considered to have Treatment Resistant Epilepsy or Drug Resistant Epilepsy (TRE/DRE). This creates a demand for an antiepileptic solution that has reportedly better efficacy and less adverse effects.
In this blog you will find historical uses of cannabis for epilepsy, current research across various epilepsy diagnoses with cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and cannabidvarin (CBDV), and additional resources for support.
Historical Uses of Cannabis for Epilepsy
Historical uses of cannabis and hemp for medicinal reasons date back thousands of years. Ancient Sumerian and Akkadian tablets found in the Middle East from as early as 1800 BCE record the use of cannabis for nocturnal convulsions. Arabic literature from around 1100 CE from Ali ibn al-Abbas al-Mayusi has been translated to state “the juice of the leaves of cannabis instilled in the nostril serves to treat epilepsy”, also suggesting that the active ingredient was tetrahydrocannabinolic acid (THCA).
The first clinical application for cannabis and epilepsy, however, is known to have taken place in the early 19th century with Irish physician William O’Shaughnessy, an army surgeon who served in India. In what can be considered the first detailed modern study of the use of cannabis-based products for anti-seizure benefits, he published his findings in 1843 after testing the behavioral effects in several mammals, fish, and birds. Among these subjects was a 40-day old baby girl with recurrent convulsive episodes. She initially responded well and after a few weeks of trials with various cannabis tinctures, taken under the tongue (sublingually), her convulsions had stopped. Several months later, O’Shaughnessy had noted that “the child is now in the enjoyment of robust health, and has regained her natural plump and happy appearance.”
From here, notice was taken across physicians in Europe and North America and by 1850 cannabis had made its way as “marijuana” into the United States Pharmacopeia, listing it as a treatment for numerous afflictions, including: neuralgia, tetanus, typhus, cholera, rabies, dysentery, alcoholism, opiate addiction, anthrax, leprosy, incontinence, gout, convulsive disorders, tonsillitis, insanity, excessive menstrual bleeding, and uterine bleeding, among others. In 1881 Neurologist Sir William Gowers wrote of the use of cannabis for seizure control in his monograph Epilepsy and Other Convulsive Disorders.
By the early 20th century, references to cannabis extractions and tinctures began to fall out in favor of Western medicine (notably phenobarbital in 1912 and phenytoin in 1937). With this, and with the soon prohibition of cannabis, the therapeutic claims and those first clinical trials took a backseat.
Despite this, chemists and pharmacologists began diving into the chemical characteristics of the active ingredients and effects on biological activity. Specifically the molecular structures of THC and CBD were investigated by Dr. Raphael Mechoulam in Israel, famously known as the “father of cannabis research”.
Researchers began to look more at CBD as potential for anti-seizure therapeutic benefits, as promising results in animal models were reviewed. Anecdotal and pre-clinical evidence increased over the years, and with the discovery of cannabinoid receptors in the late 1980’s and early 1990’s, renewed interest in the understanding of therapeutic potential of cannabinoids in how they may modulate the endocannabinoid system came about.
While smaller scale studies took place to consider the efficacy of CBD, larger scale studies began to emerge around 2015 when neurologist Orrin Devinksy and colleagues observed the antiepileptic effect of CBD among over 200 participants, ages 1-30. Their findings suggested that CBD may reduce seizure frequency and may also have an adequate safety profile in children and young adults with highly treatment-resistant epilepsy, warranting future trials.
Realm of Caring Published Research
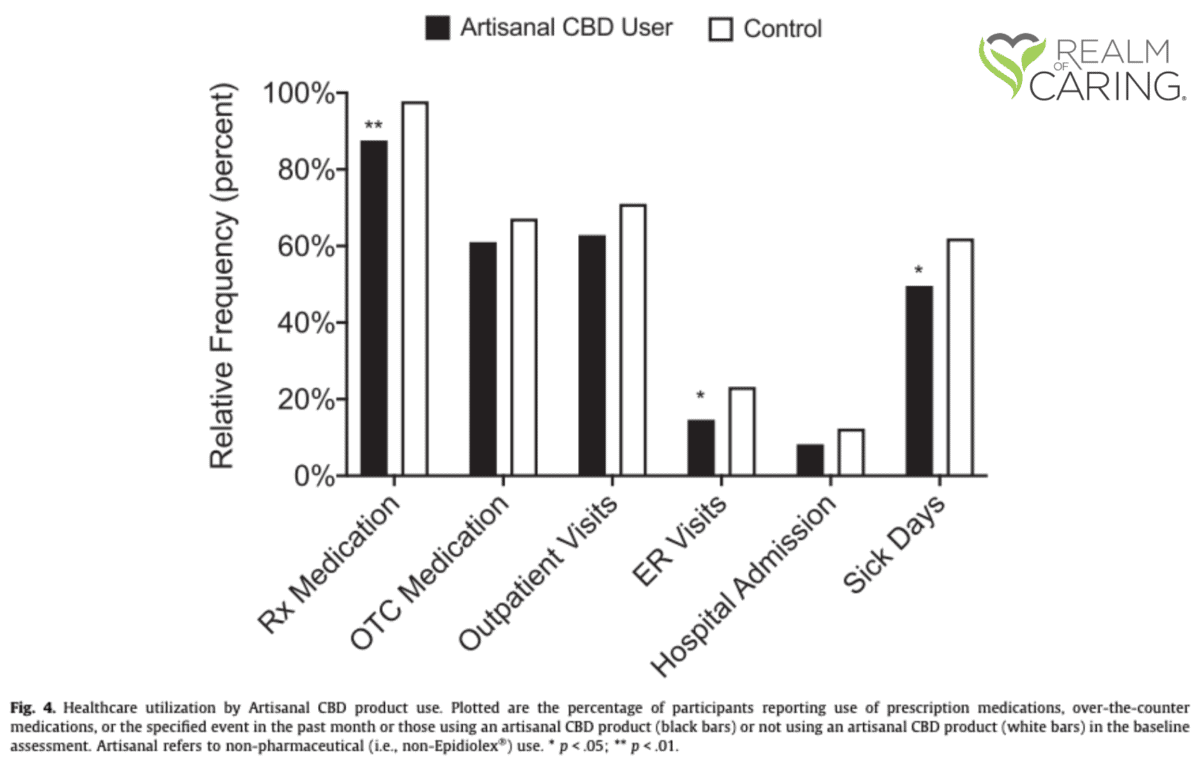
It was around this time that Realm of Caring began enrolling participants for an Observational Research Registry in collaboration with Johns Hopkins University School of Medicine. Between April 2016 and July 2020, 1,783 individuals enrolled in this online, self-reported survey. Of the enrolled individuals, 418 participants were identified to understand benefits for epilepsy where 93% had listed epilepsy as their primary medical condition with the remaining noting epilepsy as a secondary medical condition. In this sample size, there were 71 adults and 209 adult caregivers of children or dependent adults who were utilizing CBD products for medicinal use. In addition, there was a control group of 29 adults and 109 adult caregivers who were considering CBD use but had not yet begun. In-depth information from 110 participants in the study helped to conclude that the calculated median dose of CBD was 50mg/day, which equated to 1.4mg/kg/day.
The purpose of this analysis was to evaluate how CBD product use is associated with quality of life, mental health, healthcare utilization, and epilepsy-specific outcomes within a large, observational group of people with epilepsy.
Compared with controls, CBD Users had a greater health satisfaction, higher ratings of quality of life, and better scores on psychological health. Taking a deeper look, CBD Users reported lower anxiety and depression and improved sleep scores.
Other findings that were of significance relate to pharmaceutical use, healthcare visits, and caregiver burden. CBD Users had lower instances of reported prescription medication use, lower medication-related adverse effects, less ER visits, and less missing school and/or work days. In addition, caregivers of CBD Users that noted consistent use through the follow-up period reported significant decreases in the Caregiver Burden total score.
The strides that have been made in recognizing cannabis compounds for their clinical benefit to epilepsy are evident in that the U.S. Food and Drug Administration (FDA) and the European Medicines Agency have approved a CBD isolate pharmaceutical, Epidiolex, for TRE in patients with Lennox-Gastaut Syndrome (LGS) and Dravet Syndrome. Since the 2018 debut of Epidiolex, it has been approved for additional uses and further research, including that of other cannabis compounds, has taken place.
Additional, Recent Research From the Last Year
Pediatric neurologist Ellen N. Hurley published findings in early 2022 looking at five female children with Rett Syndrome and, consequently, TRE. As animal studies have demonstrated an anti-seizure effect and favorable safety profile for CBDV, the compound was used in this study to determine the safety and tolerability of it for pediatric Rett Syndrome patients. An oral CBDV solution was provided and all five witnessed a reduction in mean monthly seizure frequency.
A systematic review published in October 2022 sought to assess the effects of CBD in epilepsy patients. In conclusion, the study showed that CBD is highly efficacious both as a standalone and adjunct therapy with clobazam for controlling seizures while limiting side effects.
A study published in November 2022 analyzed thirty-five patients who were respectively prescribed CBD isolate oils, CBD broad spectrum oils, and CBD:THC combination oils. Results showed that 65.7% of patients achieved a seizure frequency of greater than or equal to 50%, demonstrating a positive signal of improved seizure frequency in children treated with cannabis-based medicinal products for treatment-resistant epilepsies.
Observational findings published in Pediatric Neurology in October 2023 support the effectiveness of purified CBD as an add-on therapy in everyday clinical practice, as researched among a mixed population of patients with DRE.
Recap: 5 Things to Consider When Starting Cannabinoid Therapy for Epilepsy
- There is published research to support benefits, which you do not have to navigate on your own. Realm of Caring features a library of these findings and a care team on standby to offer free one-on-one guidance as you read through.
- AED interactions are possible. An assessment of data suggests that changes in serum levels of AEDs taken with cannabinoid administration have been generally minor for the majority tested and may be well-tolerated. However it should be taken into consideration that several cannabinoids are processed by the body’s cytochrome P-450 (CYP450) system. Therefore it is best to consult with your doctor when co-administering cannabinoids with drugs that are also metabolized by enzymes CYP3A4 or CYP2C19.
- Patience and the biphasic response are helpful when setting expectations. Administration may be made complicated by the non-linear response of cannabinoids and we should be cautioned to assume that higher amounts of cannabinoids will yield enhanced therapeutic effects. Realm of Caring care specialists abide by the “start low and go slow” method; a strategy with a goal to find a therapeutic dose at the lowest possible amount.
- There are a variety of administration and extraction methods available. The first and most commonly used administration method would be sublingual or buccal administration with an oil or tincture. Individuals also administer by capsule or tablet, through g or j tube, and rectally as a suppository. For extractions of CBD, we have isolate, broad spectrum, full spectrum as well as formulations that include additional, natural supplements. There are also a variety of processes to extract the plant compounds. When looking for what may be most effective for you, a Realm of Caring care specialist is here to help, starting you with a quality product.
- You are not alone. In addition to the Realm of Caring care team, there is a community to support you. Many resources are available for connecting to services or more information. Reach out to our team by calling 719-347-5400 or emailing info@realmofcaring.org.