Understanding RoC’s latest published research on cannabinoid therapy and anxiety/depression.
Between April 2016 and July 2020, 538 participants were enrolled in an observational research study between Realm of Caring Foundation and Johns Hopkins University School of Medicine. Upon completion of a baseline survey, participants were invited to complete additional follow-up surveys every 3 months.
The purpose of the study was to extend prior findings with a narrow focus on participants who reported having anxiety and/or depression. The goal was to provide insight into the effects of medicinal cannabis use for symptoms of anxiety and depression.
About the Participants
The participants involved were those who were at least 18 years old and reported having anxiety and/or depression without a specific endorsement, as well as specific endorsements, including: major depressive disorder, postpartum depression, dysthymia, premenstrual dysphoric disorder, seasonal affective disorder, generalized anxiety disorder, panic disorder, social anxiety disorder, and agoraphobia.
Of the 538 participants, 368 reported current use of medicinal cannabis products at the baseline. The other 170 participants, who were considering use but had not yet initiated, served as controls. Of the participants who completed the baseline survey, 211 completed at least one follow-up assessment (145 Cannabis Users and 66 Controls).
Participants were 79% female and had a mean age of 46 years old at the baseline. The majority, at 51%, reported simultaneous diagnoses of anxiety and depression, followed by 34% reporting anxiety alone, and 15% reporting depression alone. As well, many participants, at 69%, reported a co-occurring chronic pain disorder and 36% reported use of a medication for the treatment of their anxiety and/or depression.
Product-Type and Dosing Means
Among the 74% of participants who did know the cannabinoid content of their product(s), most reported the use of CBD-dominant products (82%), followed by THC-dominant (23%), a THC:CBD balanced ratio (7%), and minor cannabinoid products such as CBG or CBN at 5%. Most individuals who reported using a THC-dominant product were also using a CBD-dominant product.
The mean CBD dose taken orally was 61mg daily, with a median of 30mg and range from 0.4mg to 1,050 mg. The mean THC dose taken orally was 2.1mg daily, with a median of 1mg and range from ≤0.01mg to 40.3mg.
Results
Cannabis Users reported lower baseline depression, significantly better past-month sleep quality, a higher overall quality of life, and lower past-month average pain compared to Controls.
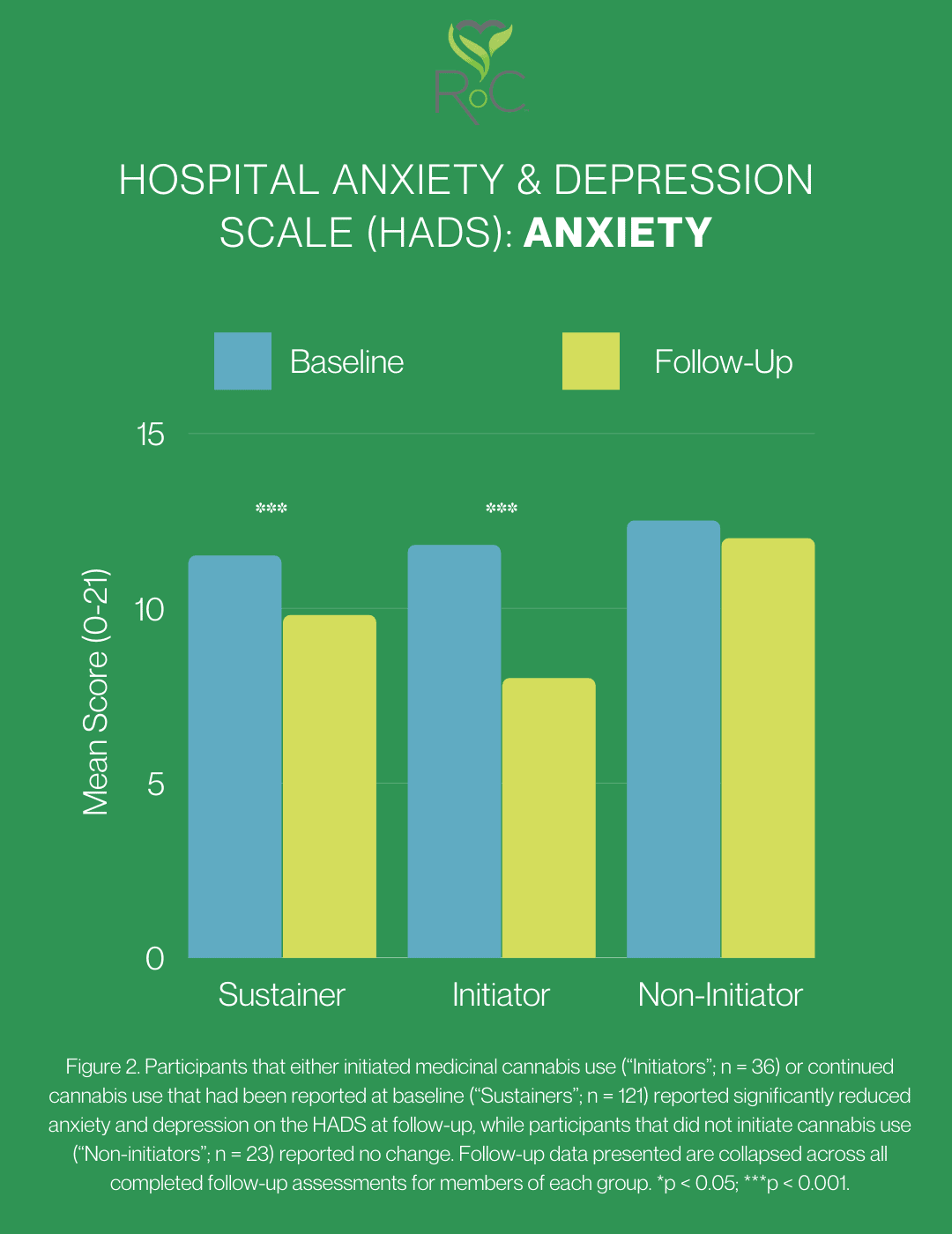
Cannabis Users did not report lower baseline anxiety, however baseline Controls who had initiated cannabis use reported a significant reduction in both mean anxiety and depression scores from baseline to follow-up surveys [evaluated using the Hospital Anxiety and Depression Scale (HADS)]. This observation was not realized among non-initiators throughout the study. A similar effect was observed among participants who sustained medicinal cannabis use throughout the study, suggesting an improvement in symptoms of anxiety and depression with both the onset of cannabis use and with extended use. The CBD doses that were used in trials that found anti-anxiety effects were greater than the average reported by participants.
Adverse Events
In response to the survey question “How has therapeutic use of cannabis harmed the participant?,” 61% of Cannabis Users reported no perceived harm or left the answer blank. Harms that were reported on included high cost (7%), social stigma/legal issues (5%), intoxication (2%), unpleasant effects associated with inhalation (2%), impaired cognition (2%), fatigue (2%), and gastrointestinal discomfort or nausea (1%). Ten participants reported worsening symptoms of anxiety with medicinal cannabis use and one participant reported worsening symptoms of depression.
Concluding Remarks
The study suggests that CBD-dominant cannabis use is associated with reduced depression among a sample of mostly female, caucasian adults. Though antidepressant effects of CBD are consistently reported in preclinical observations, it is recommended that the effects be evaluated further in placebo-controlled clinical trials under observation. Future research is necessary to confirm best dosing practices to achieve antidepressant and antianxiety effects.
◼
Antidepressant and Anxiolytic Effects of Medicinal Cannabis Use in an Observational Trial is authored by: Erin L. Martin, Justin C. Strickland, Ph.D., Nicolas J. Schlienz, Ph.D., Joel Munson, Heather Jackson, Marcel O. Bonn-Miller, Ph.D., and Ryan Vandrey, Ph.D..
For general inquiries, please contact [email protected] or call (719) 347-5400
For media inquiries, please contact [email protected]
Join our research!
Realm of Caring and Johns Hopkins University School of Medicine have developed the Observational Research Registry (ORR) to better understand medicinal cannabis use and its impact on key health outcomes including healthcare utilization, chronic pain, anxiety and depression, caregiver burden, epilepsy, and posttraumatic stress disorder (PTSD). Our registered clients provide critical information that leads to important insights into the therapeutic capabilities of medicinal cannabis. The ORR helps us develop client educational resources and may ultimately serve to legitimize the medicinal use of cannabis.
About Realm of Caring
Realm of Caring Foundation (RoC), is a 501(c)3 nonprofit organization that was established by parents in 2013 to support families who were out of medical options. By creating educational resources, conducting research, and assisting families with data-rich answers to their questions, RoC continues to be a leader in the cannabinoid (cannabis/hemp) field. RoC’s no-cost Care Team has served more than 65,000 clients worldwide and supports a network of over 2,000 medical professionals. To learn more about participating or to donate to this cause, visit www.realmofcaring.org or call 1-888-210-3772.